
Profounda receives FDA Orphan Drug Designation for miltefosine for treating Primary Amebic Meningoencephalitis (PAM)
Profounda, Inc. announced Friday that it has received, the US Food and Drug Administration’s (FDA) Orphan Drug Designation for the treatment of Primary Amebic Meningoencephalitis (PAM) with miltefosine. More...

Impavido to treat leishmaniasis receives FDA approval
PRESS RELEASE The U.S. Food and Drug Administration today approved Impavido (miltefosine) to treat a tropical disease called leishmaniasis. Phlebotomus papatasi sand flyImage/CDC Leishmaniasis is a disease caused More...

The Top 10 Infectious Disease and Outbreak News stories of 2013
2013 was a year full of impactful infectious disease stories with outbreaks of diseases not before seen in humans, diseases seen in new parts of the planet and miracle stories of survivors. Drugs not approved in More...
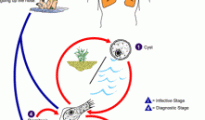
Naegleria fowleri and amoeba awareness: Personal stories and the great work from ‘Kyle Cares’ and ‘Swim Above Water’
With the summer of 2013 quickly coming to a close, the news revolving around the “brain-eating amoeba”, Naegleria fowleri, has seen everything from the 3rd known survivor of this lethal parasite in Arkansas’ More...

Naegleria fowleri: ‘Miltefosine is not the cure-all’ says ‘Kyle Cares’ founder
There has been a lot to cheer about when it comes to the treatment of parasitic meningitis caused by the amoeba, Naegleria fowleri, with the investigational drug Miltefosine, as the recent news reports on the Kali More...

Arkansas girl, Kali Hardig making great progress in her recovery, What is this experimental anti-amoeba drug?
Aug. 7 Update: It was posted on Prayers For Kali Le Ann last night–“They took Kali off the vent! She is moving and responding to questions! We are fly high on every breath she takes! Keep the prayers More...










